 https://ispwellness.asia/wp-content/uploads/2022/04/WhatsApp-Video-2022-04-07-at-3.12.09-PM.mp4
https://ispwellness.asia/wp-content/uploads/2022/04/WhatsApp-Video-2022-04-07-at-3.12.09-PM.mp4
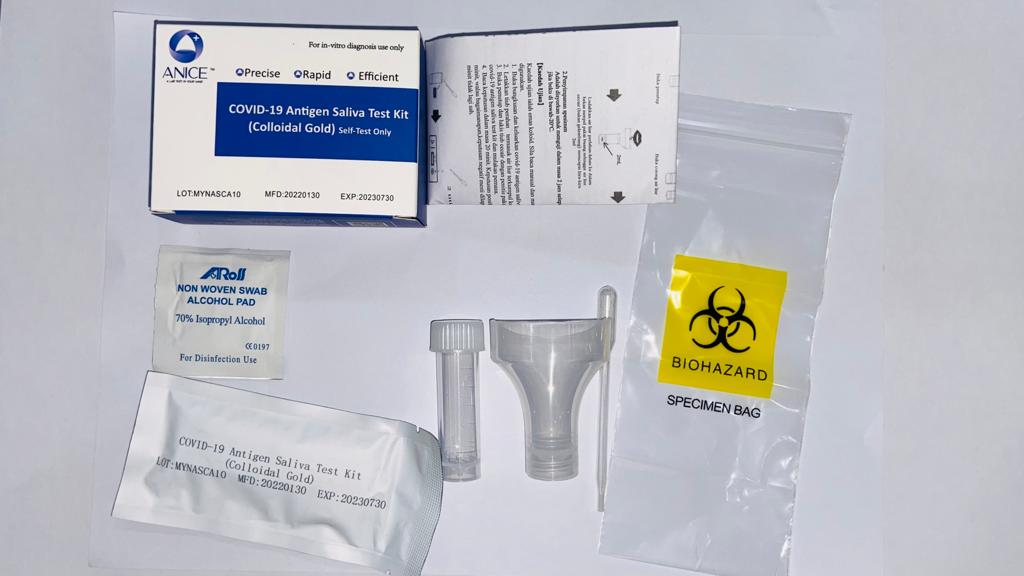
This page applies for ANICE Covid-19 Antigen Saliva test Kit (Colloidal Gold)(Self Test Only)purchased and tested in Malaysia in line with Malaysia MDA’s guidance.
MDA/GD/0059, November 2021.
Medical Device Name: ANICE Covid-19 Antigen Saliva Test Kit (Colloidal Gold)(Self-Test Only) Brand: ANICE
Identifier: ISP-CA1
Lot Number: MYNASCA10
MDA Conditional Approval Number: XXXXXXXXXXX
Before performing the test, please watch or read the instruction for use. After performing the test, Kindly complete the form below as mandatory reporting to Ministry of Health Malaysia.
Instruction for use (IFU)
Click here for get IFU
MDA Customer Record Form
The Medical Devices Authority (MDA) has instructed that customer information to be captured and shall be provided upon request by Medical Devices Authority.
MySejahtera Update
Ministry of Health of Malaysia requires individual to report the results of COVID-19 self-test kit via MySejahtera regardless of the results are positive, negative or invalid. Click here for MySejahtera update.